Why is O2 non-polar but O3 polar? In the O2 molecule, 2 oxygen atoms are joined together with a double bond between them. Each of these atoms has 2 lone pairs on each other but as there are only 2 atoms in this molecule, O2 is linear in shape. Also, the atoms are the same i.e Oxygen atom hence, there is no net dipole moment in the molecule.A oxygen molecule: O2 is bonded in a linear structure, which makes it non-polar since dipole moments are equal and point in opposite directions, thus they cancel each other out. How do you think...As an example of a polar molecule, let's take a look at water. Water is one of the most famous polar molecules, and its structure is responsible for making the molecule have a polar nature. Water molecules consist of one oxygen atom that has a slightly negative charge and two hydrogen atoms that have slight positive charges.Polar means an opposite character or tendency - in this case we are talking about charge - a positively charged pole, and a negatively charged pole. The oxygen atom is much more electronegative than the hydrogen - this means that oxygen has a greater attraction for electrons than hydrogen has.Learn to determine if O2 is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then use
is oxygen gas a polar or nonpolar molecule? | Yahoo Answers
In summation, water is a polar compound formed from two hydrogen atoms and one oxygen atom. The more electronegative oxygen atom exerts an unequal pull on the molecule's constituent electrons, causing a net dipole moment across the molecule.Silica gel, the most commonly used stationary phase, has the empirical formula SiO2. However, at the surface of the silica gel particles, the dangling oxygen atoms are bound to protons. The presence of these hydroxyl groups renders the surface of silica gel highly polar. Click to see full answer.Is CO2 polar? Carbon dioxide: Carbon dioxide is composed of one carbon atom and two oxygen atoms. Carbon is tetravalent and it forms double bonds with the two oxygen atoms by covalent bonding. ItThis is a similar mechanism to how CO2 is polar. Feel free to also check out our article about the Lewis Dot Structure for CO2 to learn more about CS2's more important counterpart. Oxygen and Sulfur have similar bonding patterns because they are both Chalcogens, having six valence electrons and being able to form two possible bonds (i.e. two
Is SO2 Polar Or Nonpolar? | Science Trends
O2 is nonpolar. Polarity is due to a difference in electronegativity. Since two atoms of the same element have the same electronegativity they can never form a polar bond. Is oxygen a polar or a...Carbon monoxide is a linear molecule, but the electronegativity difference between carbon and oxygen is significant enough to make the molecule polar. Alkynes are considered nonpolar molecules because they don't dissolve in water.With oxygen fluoride having a strong hold of electrons due to the greater pull force exerted by the compounds on the available electrons, the compound will make of itself a negatively charged molecular compound. With its unequal number of electrons, the chemical compound, oxygen difluoride, is categorized to be polar.Question = Is SO2 polar or nonpolar ? Answer = SO2 ( sulfur dioxide ) is Polar What is polar and non-polar? Polar "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.Concept: Some molecular shapes are seen as perfect and will always lead to a non-polar molecule overall. Example: Determine if silicon tetrachloride, SiCl4, is polar or nonpolar. Problem : Explain why CO2 is nonpolar, but OCS is polar?
Question = Is O2 polar or nonpolar ?Answer = O2 ( Oxygen ) is NonpolarWhat is polar and non-polar?Polar"In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have an asymmetric geometry so that the bond dipoles do not cancel each other.Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points." (Wikipedia)Polar moleculesA polar molecule has a web dipole as a result of the opposing fees (i.e. having partial certain and partial unfavorable fees) from polar bonds organized asymmetrically. (Wikipedia) http://www.school-for-champions.comPicture: water is a polar moleculeExample polar moleculesAmmonia (NH3)Sulfur Dioxide (SO2)Hydrogen Sulfide (H2S)Nonpolar moleculesA molecule may be nonpolar either when there is an equivalent sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical association of polar bonds in a more complex molecule. (Wikipedia)http://www.school-for-champions.comPicture: Carbon dioxideExample molecules non polarTolueneGasolineHelium (He)Neon (Ne)Krypton (Kr)Xenon (Xe)Hydrogen (H2)Nitrogen (N2)Oxygen (O2)Carbon Dioxide (CO2)Methane (CH4)Ethylene (C2H4)List molecules polar and non polarMolecules polarSCN- (Thiocyanate)HCO3- (Bicarbonate)BrCl3 (Bromine Trichloride)HCO3-1AsCl3 (Trichloroarsine)OCl2NO2Cl (Nitryl chloride)CH3F (Fluoromethane)H3O+ HydroniumClF (Chlorine monofluoride)ClF3 (Chlorine trifluoride)CF2Cl2 (Dichlorodifluoromethane)SeF4CH3OCH3 (Dimethyl ether)CH3CH2OH (Ethanol)NH2-BrF3 (BROMINE TRIFLUORIDE)CH3NH2 (Methylamine)CH2Br2 (Dibromomethane)HI (Hydrogen iodide)NH4NO3 (Ammonium nitrate)IF5 (Hydrogen cyanide)OH2 (Hydroxide)C2Cl2NO+ (nitrilooxonium)SBr2 ICl4+NOCH3OH (Methanol)SCl6NOBr (Nitrosyl bromide)CH4O (Methanol)ICl3 (Iodine trichloride)BrF5 (BROMINE PENTAFLUORIDE)PCl5 (PHOSPHORUS PENTACHLORIDE)CH2F2 (Difluoromethane)SeH2 (Hydrogen selenide)COS (Cobalt sulfide)OF2 (Oxygen difluoride)H2SO4 (SULFURIC ACID)H2CO (Formaldehyde)NF3 (NITROGEN TRIFLUORIDE)C2H2Br2 (Acetylene dibromide)TeF4SCN CLO3- (Chlorate)ICl5 ureaSO2Cl2 (Sulfuryl chloride)H2Se (Hydrogen selenide)NH2 XeO3SbF3CaCl2 (CALCIUM CHLORIDE)AsF3 (ARSENIC TRIFLUORIDE)C2H2 (Ethyne)BrF Cl2O IF3 (Iodine trifluoride)SH2SCl4CO (Carbon monoxide)H3OHNO3 (NITRIC ACID)N2H2NBr3 So3 2-CH3COOH (acetic acid)saltSeO2nitrogen trifluorideCCl2F2N2H4C2H5OHNoCl (NITROSYL CHLORIDE)C2H6O (Ethanol-d6)SOCl2H3O+ (Hydronium)CHF3 (Fluoroform)HClO (HYPOCHLOROUS ACID)NI3 (Nitrogen triiodide)NaCl (sodium chloride)AsH3 (Arsine)NH2Cl OCS (Carbonyl sulfide)SiCl2F2glucoseCH3N2OPoCl3 (PHOSPHORUS OXYCHLORIDE)MgCl2vinegarIOF5phosphateCHBr3 (Bromoform)ICl (IODINE MONOCHLORIDE)carbon monoxidesulfur dioxidePBr3 (PHOSPHORUS TRIBROMIDE)SF2 (Sulfur difluoride)NH3 (ammonia)SO2 (sulfur dioxide)CH2Cl2 (DICHLOROMETHANE)SF4 H2S (hydrogen sulfide)CHCl3 (CHLOROFORM)PCl3 (PHOSPHORUS TRICHLORIDE)SCl2 (Sulfur dichloride)PH3 (Phosphine)CH2O (Formaldehyde)HF (HYDROFLUORIC ACID)HBr (HYDROBROMIC ACID)NCl3 (Nitrogen trichloride)PF3 (Phosphorus trifluoride)ethyl acetateNO3- (nitrate)NO2 (Nitrogen dioxide)H2O2 (hydrogen peroxide)CH3Br (Bromomethane)cn- (cyanide)O3 (OZONE)CH3CL (Chloromethane)ammoniaOH- (Hydroxide)NO2- COCl2 (Cobalt(II) chloride)glycerolohchloroformnitrogen trichloridebenzoic acidetherno3CLF5 methylene chloridesodium chlorideCH3SHNa2SO4sodium acetateCaCO31-butanolClo-C4H10librcabr2 (Calcium bromide)CH3CN (ACETONITRILE)CH3CH2CH2OH C6H12O6LiNO3 (Lithium nitrate)MgOSeOBr2clo3c3h7ohnai (Sodium iodide)glycerinebri5BrO2ammonium chlorideCF3ClBF2ClIBrGaCl3KMnO4 (POTASSIUM PERMANGANATE)Na2S (sodium sulfide)KOH (POTASSIUM HYDROXIDE)HCOOHKBr (POTASSIUM BROMIDE)carbohydratesClO4Cl2COclo2-succinic acidH2SO3 (Sulfurous acid)sodium nitrateethyneNO2F CCL3F (Fluorotrichloromethane)ammoniumibr3fluoromethaneC2H5Cl TeCl42-propanolstarchch2clXeOF4SnCl2SCO (Carbonyl sulfide)SbBr3H2CSHC2H3O2po(oh)3Propanol1-propanolbro3-ch3ch2ch2ch3CF2Br2acetic acidanilineCFchlorine trifluoridephenolclo2potassium permanganateCH2Snitrogen monoxidech3choglassSHCOH2SOF4DNA CH3CH2NH2Malonic Acidethylene glycolisopropyl alcoholhydrogen bromideSeCl4hydrogen peroxideozonec3h6oformaldehydepo3 3difluoromethaneaspirinCHCl2rubbing alcoholfluorene or fluorenolvitamin c HClnitratedimethyl etherSClif4+Molecules non polarHBrO (Hypobromous acid)SiF4 (Silicon tetrafluoride)CH3CH3 (Ethane)N2 (Nitrogen)CO3 2- (Carbonate)Heptane-XeO4 (Xenon tetroxide)KNO3 (Potassium nitrate)CH3I (Methyl iodide)CBr4 (Carbon tetrabromide)AlCl3 (Aluminum trichloride)C6H6 (benzene)NH4BrC2H4Cl2 (dichloroethane)ligroinSO4 2- (Sulfate)C2H4SF6 (Sulfur hexafluoride)BH3 (Borane)C2F2 (Ethyne)C6H14 (HEXANE)PO4 3- (phosphate)GeH4 (Germane)PBr5 (Phosphorus pentabromide)NH4 (ammonium)waxCI4 (Tetraiodomethane)bbr3 (BORON TRIBROMIDE)H2 (Hydrogen)IF4-BeF2 (Beryllium difluoride)I2 (Iodine)GaH3 (Gallane)SeBr4 (Selenium tetrabromide)KrF4 SiBr4C2H6 (Ethane)O2 (Oxygen)biphenylPO4 3P4 (Phosphorus tetramer)SO4 2-naphthaleneXeCl2BrCl5SeF6SeO3SiH4 (silane)carbon tetrabromideAsF5 (Arsenic pentafluoride)BeH2 (Beryllium hydride)SiO2 KrF2 CH2cholesterolF2 (Fluorine)pentaneClF2C3H8PropanecyclohexanetolueneSiCl4 (Tetrachlorosilane)chlorinePF5 (Pentafluorophosphorane)BrClCO2 (carbon dioxide)CCl4 (CARBON TETRACHLORIDE)XeF2 CS2 (CARBON DISULFIDE)SO3 (SULFUR TRIOXIDE)XeF4 (Xenon tetrafluoride)BCl3 (BORON TRICHLORIDE)BeCl2 (Beryllium dichloride)CF4 (CARBON TETRAFLUORIDE)Cl2 (Chlorine)Br2 (Bromine)Hexanecarbon dioxidei3-carbon tetrachloride carbon disulfidemethaneglycinesulfur trioxidebenzophenoneoxygenAlF3 (Aluminum fluoride)beryllium dichlorideC8H18 (octane)C2Cl4PF6-XeCl4SbF5 (ANTIMONY PENTAFLUORIDE)CH3CH2CH3C5H12 (PENTANE)silanes8BF4 BF4-SeCl6BeBr2BeI2CSe2Pcl4C3H6AlH3 (Aluminum trihydride)TeO3 (Tellurium trioxide)Br-BrAsCl5octanecarbonate ionsClF4 plusacidskeroseneacetyleneC-CethaneCClCl4cbrsulfatebuttertriglyceridesgreaseberyllium chloridecarbon tetrafluoridebutane silicon tetrafluoridenitrogenICl2-brominehydrocarbonHEboron trichlorideCSsulfur hexafluoridesis2xenon tetrafluorideCH4vitamin eIonicNaBrKClNaFkf NaNO3 caoki liflicl (LITHIUM CHLORIDE)ammonium sulfateMgF2Resources: https://en.wikipedia.org/wiki/Chemical_polarityhttp://www.school-for-champions.com/chemistry/polar_molecules.htm#.WZIGddJJbccreference.comwww.quora.comanswers.yahoo.comyoutube.comgoogle.comIf the solution is mistaken, please comment in beneath article ! Question = Is O2 polar or nonpolar ? Answer = O2 ( Oxygen ) is Nonpolar Chemistry
Lewis Dot Diagram For O2 - Hanenhuusholli

Is C3N12 polar or non-polar? - Quora
Is C3N12 polar or non-polar? - Quora
Selection rules and transition moment integral - Chemwiki

Is XeF4 Polar or Nonpolar?

Is SF4 Polar or Nonpolar?

Lewis Structure Of O2 - slidesharedocs

Is O2 Polar or Nonpolar?

Lewis Dot Diagram For O2 - Hanenhuusholli

Lewis Dot Diagram For O2 - Hanenhuusholli

Is O2 Polar or Non-polar? (Oxygen Gas) - YouTube

Lewis Dot Diagram For O2 - Hanenhuusholli

O2 Lewis Dot Structure - cloudshareinfo
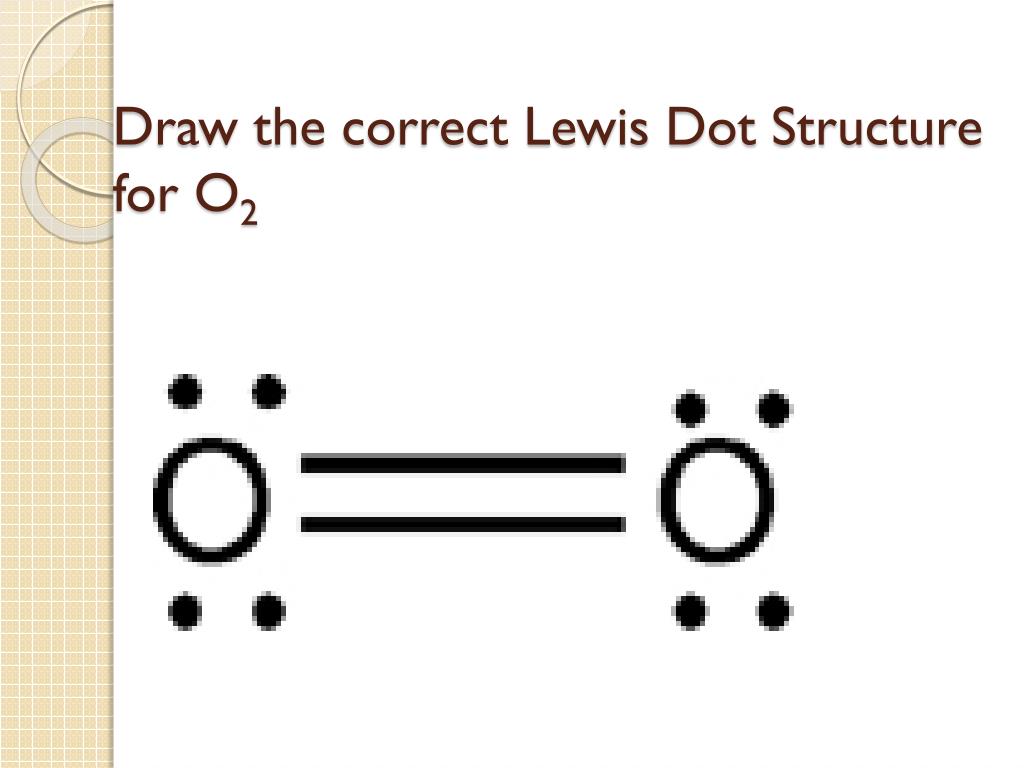
Is O2 Polar or Nonpolar?

Non Polar Covalent Compounds

Is CH2O Polar or Nonpolar?

Is O2 Polar or Nonpolar?

Lewis Dot Diagram For O2 - Hanenhuusholli

Is O2 Polar or Nonpolar?

Is NF3 Polar or Nonpolar?

Lewis Dot Diagram For O2 - Hanenhuusholli

0 comments:
Post a Comment