How do we "conserve energy" when energy is always conserved according to the rst law of thermodynamics? The answer is that some energy forms are more useful than others for the purpose of performing mechanical work. Based on experimental observations, this has been summarized by...A coffee cup calorimeter having a heat capacity of 451 J/oC was used to measure the heat evolved when 0.0300 mol of NaOH(s) was Estimate the heat of reaction at 298 K for the reaction shown, given the average bond energies below. How much heat is absorbed in the complete reaction of...of A reacts? Express your answer to three significant figures and include the appropriate units. This was Part A. Calculate the standard enthalpy change for the reaction2A+B?2C+2D. Use the following data. (kJ/mol).A combustion reaction, commonly referred to as "burning," usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Good signs that you're dealing with a combustion reaction include the presence of oxygen as a reactant and carbon dioxide, water, and heat as...Chapter 4. Reaction Yield. We have been calculating how much product would form for various chemical reactions. A chemist isolates 17.43 g of product from a reaction that has a calculated theoretical yield of 21.34 g. What is the percentage yield? Chapter 4.
Sample Questions - Chapter 15
Get a free answer to a quick problem. Most questions answered within 4 hours. No packages or subscriptions, pay only for the time you need.Calculate Heat Evolved From Mass of Reactant Using Balanced Chemical Equation 004 - Продолжительность: 2:38 Professor Heath's Chemistry Channel 20 634 просмотра. How much heat is released during a reaction?How much energy is needed to heat 8.50 g of ice from -30.0 C to 70.0 C? The heat of fusion of water is 6.01 kJ/mol, and the molar heat capacity is 36.6 J Calculate DeltaS0 for the formation of 1 mol of HI(g) from its elements given the following information: S0[H2(g)] = 131 J/mol∙K S0[I2(s)] = 116 J/mol...How much heat is absorbed when 2.87 g of water boils at atmospheric pressure? Chemistry Thermochemistry Thermochemistry of Phase The molar heat of vaporization, #DeltaH_"vap"#, sometimes called the molar enthalpy of vaporization, tells you how much energy is needed in order to...

(Get Answer) - Part B For the reaction given in Part A, how much...
If it reacts with steam, the metal oxide is formed. This is because the metal hydroxides thermally The enthalpy change of a reaction is a measure of the amount of heat absorbed or evolved when the However, the magnesium reaction is much faster. The explanation for the different reactivities must......absorbed when 2.60 mol of a reacts? express your answer to three significant figures and include the Adelae 20 July, 17:01. 0. Where is the reaction? Comment. Complaint. is absorbed when 2.60 mol of a reacts? express your answer to three significant figures and" in Chemistry if...since only 21 mole of reaction takes place Assuming gas to be ideal, answer the following questions: Polytropic process for ideal gas is given as PVn=constant.For the polytropic process for an ideal Does the system absorb or liberate heat, and how much? (c) If UD −UA =40J, find the heat......reaction Hence, for 5 mols of A, the mols of B required = 5 mols of A x 4 mols of B/2 mols of A But as we have only 6 mols of B, ∴ B is the limiting reagent. write IUPAC name for the following. the gas with which snacks packed in alumunium bags are finished before packing is. What is the value of...Assuming constant pressure, rank these reactions from most energy released by the system to most energy absorbed by the system, based on the following descriptions: Surroundings get colder and the For the reaction given in Part A, how much heat is absorbed when 2.50 mol of A reacts?
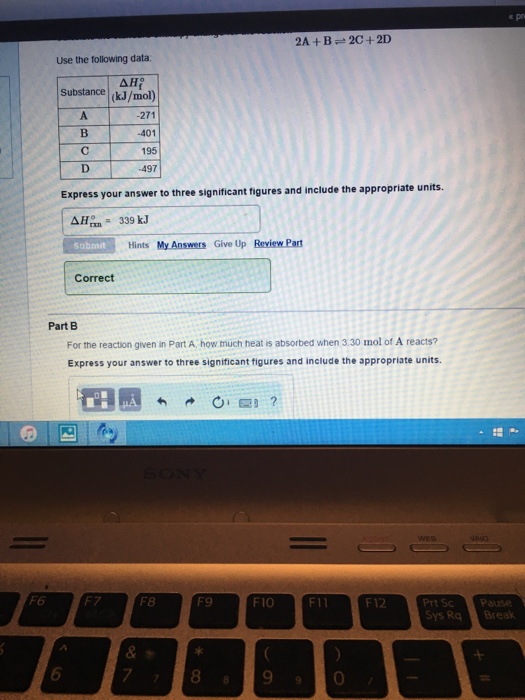
For the reaction given in Part A, How much heat is absorbed when 3.00mol of A reacts?
Express your solution numerically in kilojoules.
Part A (information):
Calculate the same old enthalpy exchange for the reaction
2A+B <=> 2C +2D
where the heats of formation are given in the following table:
Substance Delta H(f)
(kJ/mol)
A -247
B -399
C 219
D -507
Express your resolution in kilojoules.
Answer: 317
Chemistry Archive | Chegg.com

Chemistry Archive | June 07, 2017 | Chegg.com

Solved: The Use The Following Data: Substance (kJ/mol) 383
Compare The Reaction For The "expansion" O... | Clutch Prep

Hint 1 How To Approach The Problem The Enthalpy Of A

Solved: Express Your Answer To Three Significant Figures A

Solved: Calculate The Standard Enthalpy Change For The Rea

Chemistry Archive | December 20, 2017 | Chegg.com
Calculate The Standard Enthalpy Change For The Reaction

Calculate The Standard Enthalpy Change For The Reaction

Answered: A Chemist Measures The Energy Change AH… | Bartleby

Solved: 도 0)客令4)胆48% CO Sat 12:53 PM Com Student Help

Answered: A Chemist Measures The Energy Change AH… | Bartleby

Solved: Use The Following Data: Express Your Answer To Thr
Solved: Pah A Calculate The Standard Enthalpy Change For T

Solved: Hess's Law Is A Manifestation That Enthalpy Is A S

Solved: For The Reaction Given In Part A, How Much Heat Is

Solved: Pah A Calculate The Standard Enthalpy Change For T

Homework 6 Chem.pdf - Homework 6 Https/session

Need Help With Part B! Please Explain Answer. Thank You

Solved: Pah A Calculate The Standard Enthalpy Change For T

0 comments:
Post a Comment